Contraceptive Vaginal Rings
Authors
INTRODUCTION
In 2001, the first contraceptive vaginal ring (CVR) was approved for use in the United States. CVRs provide highly effective hormonal contraception with several advantages over the most commonly used method of hormonal contraception, oral contraceptives (OCs). CVRs are plastic polymer rings that contain sex steroids within them. The steroids, progestin or progesterone alone or estrogen in combination with a progestin, diffuse at a constant rate through the ring and are directly absorbed through the vaginal epithelium into the systemic circulation. Delivering steroid hormones in this way avoids the first-pass effect of hepatic metabolism and provides constant serum steroid levels, both of which lead to the greater bioavailability of the steroid hormones. This delivery system also makes longer periods of use possible, from 1–6 months of continuous use, depending on the formulation of the CVR, without the need for daily attention. A CVR containing progesterone alone has been approved for 3 months continuous use during lactation in some Latin American countries. CVRs containing progestins alone have demonstrated high efficacy, safety, and continuation rates, all comparable with OCs. Combination CVRs, containing estrogen and progestin, have been developed for cyclic use similar to combination OCs, with a scheduled withdrawal bleed every 4 weeks. Large clinical trials with combination CVRs have shown that their use effectiveness and bleeding patterns are similar to OCs.
The synthesis and preparation of orally active progestins was initially performed approximately 50 years ago. Oral preparations of progestins with and without estrogen for contraception are the most widely known, studied, and used, but the development of other methods of steroid delivery has led to new methods of hormonal contraception, including CVRs. The vaginal absorption of steroids into the systemic circulation was initially demonstrated in 1918.1 In 1966, Dzuik and Cook demonstrated the constant rate of diffusion of sex steroids through polysiloxane devices into saline solution, making the development of a CVR possible.2 Vaginal rings delivering contraceptive hormones were subsequently developed because they offered several advantages over OC preparations including constant serum steroid concentrations and longer duration of use not requiring daily attention.
The development of CVR has led to several designs with different ring materials, different steroid reservoir mechanisms, and different steroids contained within the rings. All CVRs studied or used now are flexible rings made from one of two materials: dimethyl-polysiloxane or ethylene-vinyl-acetate. Both of these are inert, nontoxic, and nonirritating materials that are well-tolerated in diameters (outer diameter: 50–61 mm; inner diameter: 46–51 mm) similar to the smallest diaphragm diameter size (60 mm).2, 3 Unlike the diaphragm, the mechanism of action is entirely dependent on the absorption of sex steroids and not any barrier mechanism. Thus, the CVR has to be in contact with the vaginal epithelium but not fitted in the vagina or over the cervix. This technique allows easy insertion and use of the CVR by the woman.3
Although different types of CVRs have been made of materials with similar properties and sizes, the design of the steroid reservoir has varied because of the steroid release requirements for contraceptive efficacy. CVRs are designed so that steroid hormones will diffuse through the CVR in a constant and steady manner for a duration of 1–12 months, depending on the amount of steroid in the CVR.4, 5 Four different steroid reservoir designs have been developed and studied: homogenous, core, band, and shell ring design. The first studied was the homogenous design, a homogenous mixture of the steroid and polysiloxane. With this design, steroids were absorbed into the systemic circulation at high levels initially, quickly decreasing over time. This inconsistent release rate of steroid hormones was related to the differential depletion of hormones in the outer and inner portions of the ring. The hormones on the outer surface of the ring would quickly diffuse and thus that portion of the ring would be rapidly depleted of hormones. Then hormones in the inner core of the ring would be released more slowly because they first had to diffuse through the outer depleted portion of the ring. Later design developments were devoted to maintaining a constant steady diffusion of hormones through the ring, and thus into the systemic circulation.
Several modifications have been made that successfully address the problem of the homogenous ring design. Core, band, and shell ring designs have steroid reservoirs in specific locations within the ring so that there is no differential depletion of hormones from the ring and hormone release is constant. The most successful ring design, the shell design, has one or more compartments containing concentrated steroid suspended within the ring between a polysiloxane or ethylene-vinyl-acetate core and a rate controlling outer layer. Compared with the other designs, this configuration provides a more constant release of hormones for up to 6 months.4, 6 CVRs of each design contain steroid hormones of different formulations, either progesterone alone, progestin alone, or progestins in combination with estrogen. Clinical trials of these various CVR formulations have been reported since 1970.7
CONTRACEPTIVE VAGINAL RING CONTAINING PROGESTIN ALONE
The first clinical trial demonstrating contraceptive efficacy from a vaginal ring contained medroxyprogesterone acetate alone. Progestin-only CVRs are used for durations up to 6 months, either cyclically or continuously, removing the ring only as desired for rinsing after a bleeding episode or briefly for intercourse. Although not available for use in this country, progestin-only CVRs could provide effective contraception to women for whom estrogen is contraindicated without the need for strict adherence to a daily dosing schedule (as is the case with progestin-only oral contraception).
That first clinical trial of a CVR evaluated a homogenous CVR containing 100-mg medroxyprogesterone acetate.7 This CVR was worn for 21 days and then removed for 7 days, in the cyclic manner of most OC formulations. Ovulation suppression in this trial occurred in 94% of observed cycles with minimal breakthrough bleeding (<4% of cycles). This CVR was not investigated further because of safety concerns related to medroxyprogesterone acetate, concerns that subsequently have not been proven. Norethindrone acetate and norgestrel were the next progestins evaluated. In a comparative trial of homogenous rings containing either norethindrone in doses of 50–200 mg or norgestrel in doses of 10–100 mg used in the same cyclic manner for up to 6 months, CVRs with various doses of either progestin did not consistently suppress ovulation, had breakthrough bleeding, and had an unpleasant odor when exposed to blood.8
Further development then concentrated on other ring designs and other progestins to improve efficacy, safety, and acceptability. The World Health Organization (WHO) developed a core design CVR containing 5 mg of levonorgestrel that released 20 μg/day.9 This ring was worn continuously for a duration of 3 months. Ovulation was not consistently suppressed with this ring but this was not the sole contraceptive mechanism. Thickened cervical mucous and local effects on the endometrium together with ovulation suppression made this ring very effective. During an observation period of 8176.7 women-months of use, the pregnancy rate was 4.5 per 100 women-years. The continuation rate at 1 year was approximately 50%, and menstrual disturbances were the most commonly cited reason for discontinuation (17.2%).9 The disturbance in bleeding patterns associated with the use of this CVR was most commonly frequent or irregular bleeding patterns rather than a prolonged duration of bleeding.10 This finding was confirmed in another trial of the same CVR designed specifically to measure the amount of uterine blood loss.11 Despite the CVR association with frequent or irregular bleeding patterns, compared with baseline, uterine blood loss was decreased in CVR users compared with that occurring in ovulatory cycles. However, a preliminary report of a clinical trial suggested an association between the use of this CVR and the development of vaginal lesions.12 Further research concentrated on other formulations and designs as a result of this possible association.
A CVR containing progesterone alone is currently approved for contraceptive use during lactation in some Latin American countries where the incidence of breastfeeding is high. The core-design polysiloxane CVRs containing progesterone have been shown to be highly effective for lactating women, but not normally cycling women. Two CVRs releasing 5–10 mg per day of progesterone and ranging in size from 58–61 mm in diameter demonstrated very high efficacy during lactation compared with a control group using copper intrauterine devices (IUDs).13 Only one pregnancy was observed in 739 women-months of CVR use, and this was associated with imperfect use of the method.13 Bleeding patterns were significantly different between the treatment groups, with higher rates of amenorrhea in CVR users than the IUD users. For the outcomes of continued breastfeeding infant growth and infant well-being, the CVR users and the IUD users had similar rates of each, demonstrating the safety of the CVR during lactation.
Two later studies of CVRs releasing 10 mg of progesterone daily in postpartum lactating women confirmed these findings. One was a large multicenter international trial of a homogenous CVR, and the other was a smaller trial in China of a core CVR.14, 15 Both CVRs had the same dimensions, an outer diameter of 58 mm and an inner diameter of 49.6 mm. In both trials, the CVR was worn continuously after delivery and replaced every 3 months for a total of 12 months, and both trials used a nonrandomized control group of copper IUD users. In the larger trial of the homogenous type CVR, the 1-year pregnancy rates in the CVR users was 1.5 per 100 women-years, which was not significantly different than the IUD users.15 There were no pregnancies in the smaller trial of the core-type CVR.14
In these studies, the earlier findings regarding bleeding patterns were confirmed; bleeding patterns were markedly different in the CVR users compared with the IUD users. Again, CVR users were more likely to experience amenorrhea than IUD users. This is an expected finding because the CVR provides both ovulation suppression and endometrial suppression, neither of which is accomplished with a copper IUD. After 6 months of CVR use, approximately two-thirds of CVR users were amenorrheic, and at 1 year 45% of CVR users were amenorrheic.15 The rates of amenorrhea in the CVR users were significantly greater than those of IUD users; less than half of the IUD users were amenorrheic at 6 months, and 16% at 1 year. Even though CVR users experienced fewer bleeding episodes than IUD users, they were twice as likely to discontinue use because of an unacceptable bleeding pattern. Compared with IUD users, CVR users were also more likely to discontinue use because of vaginal symptoms. This was not directly related to more local disruption or irritation by the CVR compared with the IUD. The incidences of macroscopic vaginal lesions, cervical lesions, and infectious disease were higher for IUD users than CVR users.15
The greater discontinuation rates for vaginal or menstrual complaints was consistent with the continuation rates overall which were higher for IUD users than CVR users. In these two studies, the 1-year continuation rates for CVR users were 23.5–34.6 per 100 women-years compared with 88.1–97.7 for IUD users. Reasons for discontinuation related to the method of contraception were usually related to either use-related problems (expulsion, unpleasant experience for the CVR) or weaning for CVR users. For both groups women did not wean before 6 months and the continuation rate for the CVR at that time was almost 50%.15 All investigations of the progesterone CVR confirmed the safety of the CVR for the nursing child as there was no difference in infant growth or well-being between CVR and IUD users in any study, and there were no reported discontinuations for reasons related to the infant. Because of the strong evidence for the safety, efficacy, and acceptability of this CVR during lactation it has been approved for clinical use during lactation in some Latin American countries.
CONTRACEPTIVE VAGINAL RING CONTAINING ESTROGEN IN COMBINATION WITH A PROGESTIN
Although estrogen is not required for contraceptive efficacy of hormonal methods of contraception, the combination of estrogen and progestin in OCs increased the effectiveness of the method and improved bleeding patterns compared to progestin only OCs. CVR formulations that contain and release both a progestin and estrogen have cyclic patterns of use that mirror combination OCs. The CVRs are worn for 3 weeks and then removed for 1 week to allow withdrawal bleeding. In combination CVRs, the addition of estrogen combined with a higher dose of progestin result in greater effectiveness compared with progestin-only CVRs, similar to combination OCs. In addition, the estrogen component maintains the endometrium and prevents breakthrough bleeding.
Several formulations using different progestins have been investigated for clinical use. Levonorgestrel and estradiol combined in a CVR demonstrated high efficacy, excellent bleeding patterns, and high continuation rates. Clinical studies of two CVRs of different sizes containing levonorgestrel and estradiol confirmed almost complete ovulation suppression with use. The 1-year pregnancy rate was 2.4 per 100 women for a 50-mm CVR and 1.4 per 100 women using a 58 mm CVR.16, 17 Continuation rates were also high, with approximately half of CVR users still using the method at 1 year, more than OC users in a randomized trial comparing the two groups.17 There were no clinically significant changes in the lipid panel with this CVR in several studies.18, 19, 20, 21 However, concern regarding the effect on lipids by CVRs containing levonorgestrel and estradiol arose in a study comparing CVRs with five different progestins; progesterone, levonorgestrel, norethisterone, medroxyprogesterone acetate, megestrol acetate.22 All but the progesterone CVR were combined with estradiol. In this trial, use of the CVR containing levonorgestrel and estradiol was associated with a significant decrease in HDL levels and in the LDL-to-HDL ratio, and an increase in LDL levels.22 These changes were not observed with the other CVRs studied.
Animal data on atherosclerotic disease corroborated the association of this CVR with adverse changes in lipids. In a comparative trial, macaques treated with a CVR containing levonorgestrel and estradiol for 2 years had atherosclerotic changes that were greater than those in the controls.23 Therefore, despite the high efficacy, acceptability, and desirable bleeding patterns associated with this CVR, further study of combined hormonal CVRs were directed to other formulations. The lipid effects seen with this combination CVR are presumed to be caused by the use of a 19-nortestosterone derivative progestin (levonorgestrel) combined with a low-potency estrogen (estradiol). Later developments of combination CVRs would include the more potent estrogen found in combination OCs, ethinyl estradiol.
Norethindrone acetate, a progestin used in OCs, was paired with ethinyl estradiol in a core-design CVR. A CVR releasing 650 μg/day of norethindrone acetate and 30 μg/day of ethinyl estradiol was associated with serum levels of both norethindrone and ethinyl estradiol comparable with women ingesting an oral contraceptive containing 30-μg ethinyl estradiol and 1.5-mg norethindrone acetate.24, 25 In a comparison of CVR users and OC users with these formulations, serum progesterone levels remained low, indicating inhibition of luteal activity in both groups. Side effects were reported with similar frequency by both groups, except nausea, which was greater for CVR users. The lipid profile of CVR users differed from OC users. CVR users had a significant increase in triglycerides and HDL levels. Other serum lipid levels including LDL and total cholesterol were unchanged with the use of this CVR.
Another formulation releasing 1 mg of norethindrone acetate per day and 20 μg of ethinyl estradiol per day was designed to be used cyclically for 1 year.26, 27, 28 This CVR had an outer diameter of 58 mm and inner diameter of 50.4 mm. This CVR was not associated with the same high rate of ovulation suppression as the previous formulation.26 Despite this, two large clinical trials demonstrated excellent contraceptive efficacy. There were no pregnancies in 654 women-months of use of this CVR in one study and one pregnancy in 831 women-months of this CVR use in another.26, 28
This CVR was designed, like other combination CVRs, to be used in a cyclic fashion to provide regular withdrawal bleeding. Similar to previous trials of combination CVRS, most episodes of bleeding occurred during the scheduled withdrawal week. Episodes of intermenstrual bleeding (bleeding with the CVR in place) were uncommon, occurring in less than 2% of treatment days and less than 20% of treatment cycles.26 Furthermore, the breakthrough bleeding that did occur, usually began immediately before the time of scheduled CVR removal. Amenorrhea was rare. Nearly all subjects experienced a scheduled withdrawal bleed during each cycle. Other side effects and outcome parameters were similar to previous studies of combination CVRs. Body weight was not affected by this CVR. The changes observed in serum lipid levels were comparable with the trial of the earlier norethindrone combination CVR. After 1 year of CVR use, average serum levels increased for all lipid parameters. On average, serum triglycerides increased 20–33%, HDL increased 2–12%, total cholesterol 3–9% and LDL 1–17% at each study site. None of these changes was considered clinically significant. Despite the high efficacy, acceptable bleeding patterns, and safety of this CVR, it was not developed for clinical use.
The Population Council, which is responsible for the development of many of the CVR formulations and many other contraceptive methods available today, has studied a novel progestin, 17-acetoxy-16 methylene-19 norprogesterone (Nestorone). Nestorone is unusual because although it has pharmacologic activity similar to other progestins, it has no oral bioavailability. It does have good parenteral bioavailability. When absorbed through the vaginal epithelium, Nestorone demonstrated contraceptive efficacy and minimal androgenicity. When combined with ethinyl estradiol, a CVR with Nestorone was designed for monthly cyclic use to be used for a 3-month period. A CVR containing 60 mg of Nestorone and 122 mg of ethinyl estradiol was associated with complete ovulation suppression and no significant change in serum lipids in 72 women-months.29
Subsequently, a comparative trial of a core-design CVR was performed for several formulations. This core-design CVR had an outer diameter of 58 mm and inner diameter of 50.4 mm and was designed for cyclic use over a 3-month period. In the comparative trial, four formulations were evaluated; progestin-only formulations of Nestorone or levonorgestrel acetate each releasing 100 μg/day or each of these progestins combined with ethinyl estradiol releasing 30 μg/day.30 As occurred in trials of other progestin-only and combination CVR formulations, ovulation suppression was better and bleeding patterns were more predictable with the two combined formulations than the two progestin only formulations. The combined Nestorone/ethinyl estradiol CVR was associated with the most predictable bleeding pattern. The five women who received this CVR reported no episodes of breakthrough bleeding for the study duration, a total of 15 cycles. Mean total cholesterol levels increased for all CVR formulations, but no formulation was associated with a clinically significant increase. All Nestorone containing CVRs were associated with increased triglyceride levels. Levonorgestrel-containing formulations, in contrast, were associated with a decrease in triglyceride levels. Nestorone combination CVRs have promise as a safe, effective, and acceptable method of contraception. Phase III trials of a combined Nestorone ethinyl estradiol ring are currently ongoing.
One CVR has recently been marketed for contraceptive use in the United States. Under the trade name NuvaRing, the core-design CVR contains etonogestrel and ethinyl estradiol. The material of the CVR is ethylene-vinyl-acetate, which is a polymer that is different than polysiloxane but similar to polysiloxane in that it is nontoxic and nonirritating. This CVR measures 54 mm in outer diameter and 50 mm in inner diameter and releases 120 μg/day of etonogestrel and 15 μg/day of ethinyl estradiol.
Etonogestrel, or 3-keto-desogestrel, a gonane progestin, is the active metabolite of desogestrel. This progestin has demonstrated efficacy and safety in a single-rod subdermal implant system, Implanon. Etonogestrel, in this implant, does not have atherogenic or thrombotic properties in studies evaluating markers of each.31, 32 Studies evaluating the effect of the Nuvaring on lipid metabolism have confirmed minimal effects on lipid profiles. One multi-center open-label randomized trial comparing the effects of the Nuvaring and a combined oral contraceptive containing ethinylestradiol 30 mcg and levonorgestrel 150 mcg on lipid profile over 6 cycles showed that the etonogestrel CVR did not effect total cholesterol, increase in HDL2, and decreased LDL after 3 cycles. The COC was not associated with a significant net effect on total cholesterol, but was associated with a decrease in HDL, HDL2, and HDL3, and increase in LDL after cycles 3 and 6. Again, the lower androgenicity of the etonogestrel was reflected in the minimal impact on lipid profiles.33 A subsequent open-label randomized trial comparing metabolic effects of the Nuvaring to an oral contraceptive pill containing 20 mcg ethinyl estradiol and 100 mcg levonorgestrol confirmed the minimal impact of Nuvaring on lipid profile. Nuvaring was found to have significantly less of a reduction in insulin sensitivity index compared to the ethynylestradiol containing COC.34
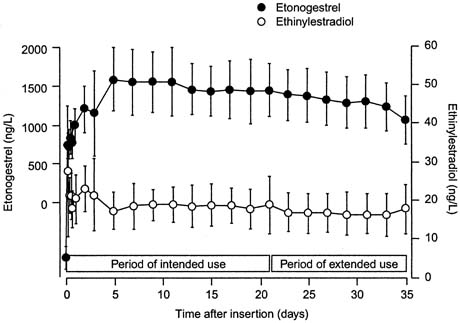
In a randomized trial of CVR users and OC users ingesting a combined oral contraceptive containing 150 μg of desogestrel and 30 μg of ethinyl estradiol, serum levels of 3-keto-desogestrel and ethinyl estradiol were compared.35 Similar to pharmacologic data with other CVR formulations, therapeutic levels of both hormones were reached by day 3 of use (Fig, 1). Also consistent with other CVR formulations, the peak serum levels of both hormones were lower in the CVR group compared with the OC group (Table 1). The peak serum levels of 3-keto-desogestrel in the CVR users were less than half those measured in combined oral contraceptive users, and peak serum levels of ethinyl estradiol were approximately one-fourth the levels of OC users. Also consistent with the pharmacokinetics of other ring formulations was the greater bioavailability (the area under the curve) of 3-keto-desogestrel (etonogestrel) in CVR users than combined OC users. The bioavailability of ethinyl estradiol was similar between the two groups.
Table 1. Pharmacokinetic parameters of a contraceptive vaginal ring compared with a combination oral contraception (means ± standard deviations)
| Etonogestrel/Ethinyl Estradiol CVR (N = 16) | Desogestrel/Ethinyl Estradiol Oral Contraceptive (N = 16) | |
| Etonogestrel | ||
| Peak serum concentration (ng/L) | 1716 ± 445 | 4273 ± 830 |
| Bioavailability (%) | 102.9 ± 12.8 | 79.2 ± 7.7 |
| Ethinyl estradiol | ||
| Peak serum concentration (ng/L) | 034.7 ± 17.5 | 124.9 ± 46.3 |
| Bioavailability (%) | 055.6 ± 12.9 | 053.8 ± 17.6 |
(Adapted from reference 35.)
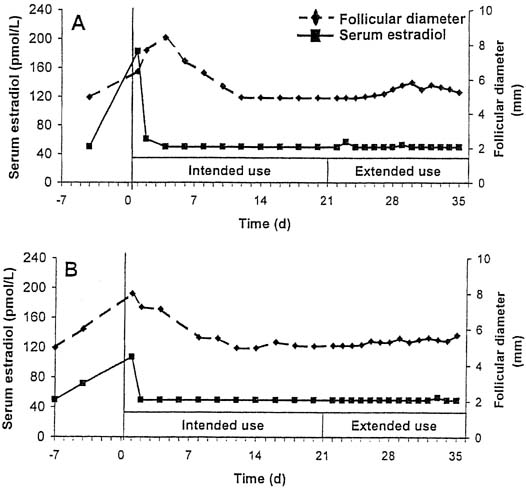
Although designed for cyclic use, with a withdrawal bleed in the fourth week after 3 weeks of use, this CVR provides serum levels of hormones that suppress ovulation for a period of up to 5 weeks. In a small study of extended use, subjects using the ring for a continuous 5-week period did not have a significant decrease in either serum etonogestrel or ethinyl estradiol levels.35 Ovulation inhibition was determined by measurement of follicular growth, serum progesterone, estradiol, and luteinizing hormone levels. Complete inhibition of ovulation was demonstrated with extended use of the CVR for up to 5 weeks (Fig, 2).36
A large, multicenter, international study demonstrated the safety and efficacy of this CVR.37 A total of 1145 women used this CVR for up to 13 cycles, for a total of 12,109 cycles observed. Only six pregnancies occurred in this study, yielding a failure rate of 0.65 per 100 women-years, confirming high efficacy of the CVR. Regular, scheduled withdrawal bleeding (during the ring-free week) was observed in almost all treatment cycles (97.9–99.4%). A minority of subjects reported unscheduled bleeding or spotting with the CVR in place. Unscheduled bleeding or spotting was reported in only 2.6–6.4% of each of the 13 treatment cycles, most reporting irregular spotting (Table 2).
Table 2. Incidences (percentages) of bleeding as a proportion of intention-to-treat evaluable cyclesa
| Irregular bleeding (breakthrough bleeding/spotting): | 2.6–6.4 |
| Withdrawal bleeding | |
| Absence | 0.6–2.1 |
| Earlyb | 5.4–7.7 |
| Early with spotting only | 2.8–5.4 |
| Latec | 20.4–27.3 |
| Late with spotting only | 16.5–21.4 |
aPercentages apply to cycles 1–12 for incidences of late withdrawal bleeding, and to cycles 1–13 otherwise.
bWithdrawal bleeding started while the CVR was in place.
cWithdrawal bleeding continued after the CVR was replaced.
(Reprinted from reference 37.)
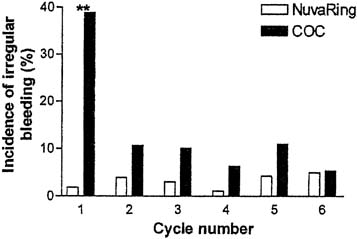
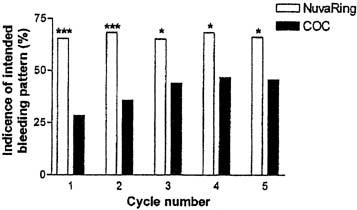
In a comparative trial of this CVR and an OC containing levonorgestrel 150 μg and ethinyl estradiol 30 μg, the CVR was equally effective and had a better bleeding pattern than the OC.38 There were no pregnancies during treatment among either the 126 OC users or the 121 CVR users. CVR users were more likely to experience regular withdrawal bleeding on schedule and less likely to experience irregular or unscheduled bleeding than OC users (Figs. 3 and 4). The incidence of hormonal side effects were also similar between the CVR and OC users, without a significant difference between them (Table 3). All treatment-related hormonal side effects were reported by less than 10% of subjects, with decreased libido (8.3%), nausea (5.0%), breast tenderness (4.1%), and vaginitis (4.1%) most commonly reported by CVR users. In addition, cervical cytology was not significantly different after treatment for either group.
Table 3. Proportion of subjects who reported AEs that were possibly treatment-related (occurring in ≥ 2% of subjects in either treatment group)
| NuvaRing | COC Users | |
| AE | N (%) | N (%) |
| Acne | 02 (1.7) | 3 (2.4) |
| Breast tenderness | 05 (4.1) | 5 (4.0) |
| Decreased libido | 10 (8.3) | 0 (0.0) |
| Depression | 00 (0.0) | 6 (4.8) |
| Device-related events* | 03 (2.5) | NA (.0) |
| Headache | 04 (3.3) | 3 (2.4) |
| Leukorrhea | 03 (2.5) | 0 (0.0) |
| Nausea | 06 (5.0) | 4 (3.2) |
| Nervousness | 03 (2.5) | 2 (1.6) |
| Weight increase | 04 (3.3) | 2 (1.6) |
| Vaginal discomfort | 03 (2.5) | 0 (0.0) |
| Vaginitis | 05 (4.1) | 2 (1.6) |
NA, not applicable
*Comprising foreign body sensation, coital problems, and expulsion.
(Reprinted from reference 38.)
A later study comparing the ethinylestradiol pharmacokinetics of the etonogestrol containing CVR, the transdermal patch, and COC containing 30 mcg ethineyl estradiol and 150 mcg desogestrel after 21 days of treatment. The area under the curve of ethinyl estradiol concentration-versus-time showed CVR ethinyl estradiol 3.4 times lower than the patch (p<0.05) and 2.1 times lower than COC (p<0.05). Peak concentration of ethinyl estradiol in COC was 4.5 times greater than CVR (p<0.05) and patch was 2.8 times higher than CVR (p<0.05). Additionally the CVR showed the least amount of variation in ethinyl estradiol levels compared to the patch and the COC. Treatment related adverse events were more frequent in the patch group (n = 57) compared to CVR ( n = 18), and COC (n = 1). The most common treatment related event was headache in the three groups. Adverse events attributed to estrogen - nausea and breast tenderness - were more common with the patch (3 of 8 and 5 of 8 subjects respectively) than with the CVR (1 of 8 and 0 of 8 subjects respectively) or with the COC (0 subjects).39
The Food and Drug Administration approved this CVR releasing 120 μg/day of etonogestrel and 15 mcg/day of ethinyl estradiol for use as a contraceptive in 2001. This CVR became available for use in 2002 with the trade name Nuvaring. The addition of a CVR to the already available reversible forms of contraception has greatly expanded the contraceptive choices for women in the United States.
CLINICAL CONSIDERATIONS
The NuvaRing CVR has similar labeling precautions as other combination OCs. Contraindications to its use listed in the product labeling are: thrombophlebitis or thromboembolic disorders; past history of deep vein thrombophlebitis or thromboembolic disorders; cerebral vascular or coronary artery disease (current or history); valvular heart disease with complications; severe hypertension; diabetes with vascular involvement; headaches with focal neurological symptoms; major surgery with prolonged immobilization; known or suspected carcinoma of the breast or personal history of breast cancer; carcinoma of the endometrium or other known or suspected estrogen-dependent; neoplasia; undiagnosed abnormal genital bleeding; cholestatic jaundice of pregnancy or jaundice with prior hormonal contraceptive use; hepatic tumors (benign or malignant), active liver disease; known or suspected pregnancy; heavy smoking (>15 cigarettes per day) and over age 35 years; and hypersensitivity to any of the components of NuvaRing.
The package labeling also contains instructions on how to insert and use the NuvaRing. Patients are advised to insert the ring in a comfortable position, either standing with one leg up on a step, squatting, or lying down. The ring is compressed and inserted into the vagina with one hand. The ring is then left in place for 3 consecutive weeks and then removed either by inserting the index finger into the vagina and hooking the rim or by inserting the index and middle fingers and grasping the rim, and then pulling the ring out. After a 1-week ring-free interval, a new ring is inserted. After use, the ring should be wrapped in its foil pouch before discarding and it should never be discarded in the toilet or other plumbing, for environmental considerations.
A case report of incorrect CVR placement underscores the importance of assessing patient understanding of insertion technique. The case is of a 22 year old woman gravida 2, para 2, who presented with persistent dysuria after CVR placement two months prior, described as ground glass coming from her urethra. The patient had been seen several times her symptoms had not been improved by treatment for urinary tract infections. A CT scan for persistent symptoms showed a ring-like translucency in the bladder. A urologist confirmed the Nuvaring placement in the bladder and removed it using office cystoscopy.40 Thus, having the patient try to insert and remove the CVR in the office might help clarify any questions or concerns which a patient may develop while inserting the ring at home.
If the patient and/or partner desires, the ring may be removed for intercourse. When the ring is removed for intercourse or spontaneously expels, the ring should be reinserted within 3 hours to maintain contraceptive efficacy. If the ring is removed for more than 3 hours during the 3-week use period, it should be reinserted immediately and a backup method of contraception should be used for 1 week. Before reinserting the same ring, it should be rinsed in water, and care should be taken not to use hot water or soaps of any kind, because this may interfere with steroid hormone release.
Women should be instructed to insert the first ring during the first 5 days of the menstrual cycle and use a backup method of contraception for the first 7 days of use. If a patient is switching from combination OCs, the first ring may be inserted anytime within the placebo week of the OC pill pack. In this scenario, no backup method of contraception is required. When switching from progestin-only methods, patients should insert the first ring immediately after the last OC pill, immediately after a contraceptive implant is removed, immediately after an IUD is removed, or on the date a contraceptive injection was scheduled. A backup method of contraception is recommended for the first 7 days of ring use for these scenarios.
After pregnancy, patients may insert the ring at a time similar to that of OC initiation. After a complete first-trimester abortion, women may insert the ring within the first 5 days and do not need to use a backup method of contraception. If, however, more than 5 days has elapsed, a backup method of contraception is recommended for the first week of use. Patients are advised to insert the ring 4 weeks after delivery or a second-trimester abortion and use a backup method of contraception for the first week of use. The NuvaRing is not recommended for use during lactation.
Bleeding Patterns
CVRs have been designed to provide very different patterns of uterine bleeding, either amenorrhea or predictable withdrawal bleed during a ring-free week. CVRs containing progesterone or progestin alone are worn continuously without a ring-free period to achieve contraceptive efficacy and amenorrhea. The progesterone containing CVR when used by lactating women achieved amenorrhea for almost half of all subjects at 1 year of use.15 This incidence was approximately three-times greater than lactating women using a copper IUD. Combined hormonal CVRs were all designed to achieve the regular monthly withdrawal bleeding pattern of combination OCs. This cyclic pattern of bleeding was meant to mimic the menstrual cycle and signal the absence of pregnancy. The bleeding patterns associated with either OCs or CVRs, however, are different from a natural menstrual cycle; the bleeding is lighter because of endometrial suppression, and there is a higher incidence of breakthrough bleeding (bleeding that occurs apart from the hormone-free week).
The most common reported menstrual disturbance in CVR studies is breakthrough bleeding. Unscheduled bleeding or spotting is reported in all studies of all formulations of the CVR. Like OCs, unscheduled bleeding occurs with greater frequency in CVRs containing progestin-only than with combined hormonal CVRs. When directly compared, CVRs containing either levonorgestrel or Nestorone were both associated with breakthrough bleeding in 40% of cycles, whereas CVRs containing ethinyl estradiol combined with either levonorgestrel or Nestorone were associated with breakthrough bleeding in less than 18% of cycles.30 In a large WHO-sponsored clinical trial of a levonorgestrel CVR, on average, subjects experienced breakthrough bleeding or spotting every 3 weeks.10 During 8177 women-months of CVR use, subjects reported an average of 16 to 18 bleeding or spotting days per 90-day reference period, with a mean length of 4 to 5 days and a mean interval (no bleeding or spotting) of 17 to 18 days. The amount, frequency, or duration of breakthrough bleeding or spotting in this study was not associated with the type of contraception (hormonal, barrier, or copper IUD) used by the subject before study entry.
Studies of the CVR containing etonogestrel and estradiol have confirmed the bleeding patterns reported in earlier studies of other combination CVRs. A large clinical trial of the etonogestrel and ethinyl estradiol-releasing CVR observed 1145 women for 12,109 cycles of use.37 In this trial, regular scheduled withdrawal bleeding was almost universally experienced, subjects reported that withdrawal bleeding beginning 2–3 days after ring removal lasted 4–5 days on average. Breakthrough or unscheduled bleeding occurred uncommonly. Subjects noted unscheduled bleeding in less than 7% of the cycles. Irregular bleeding or spotting usually occurred close in time to the withdrawal bleed, either just before ring removal (5.4–7.7% of cycles) or, more commonly, bleeding that continued after the ring-free week when the ring was re-inserted (20.4–27.35 of cycles) (see Table 2). A later study of the same CVR confirmed superior cycle control achieved with use of this CVR compared to an OC (see Figs. 3 and 4).38 A multicenter open-label, randomized trial comparing cycle control of Nuvaring versus an oral contraceptive ring containing 30 mgc ethinyl estradiol and 3 mg drospirenone over 13 cycles found a trend towards superiority of the CVR with regards to breakthrough bleeding/spotting for all cycles except cycles 11 and 12, though this did not reach statistical significance. Intended bleeding was significantly higher in CVR group compared to COC group for all cycles ( p < 0.01).41
All formulations of CVRs, whether they contain only a progestin or also contain estrogen, decrease mean uterine blood loss compared with no treatment. In a study of the Nesterone and ethinyl estradiol-containing ring, mean uterine blood loss was decreased up to 63% compared with nontreatment cycles.42 This substantial decrease in blood loss is related to the pattern of bleeding achieved with a CVR rather than the increase in breakthrough bleeding associated with a CVR. Although progestin only CVRs are associated with more breakthrough bleeding, they are also associated with a higher rate of amenorrhea than oral contraceptives or IUDs. Furthermore, when unscheduled bleeding occurs, the total duration or amount of bleeding is not usually increased overall. This finding accounts for the overall decrease in menstrual blood loss and improvement in hemoglobin measurements despite more breakthrough bleeding episodes with CVRs.10, 22
Extended Use
In light of use of extended regimens of COCs, studies have been performed evaluating alternative extended regimens of the combined vaginal ring containing ethinyl estradiol and etonogestrel. One open-label, randomized trial evaluated bleeding patterns and tolerability of four regimens cycles: monthly (28 day cycle), every other month (44 day cycle), every third month (91 days), and continuous (364 days) over one year. A total of 429 women were randomized and 289 (67.4%) completed the one year study follow up. Study completion rates were higher in the shorter cycles and more women discontinued the study due to unacceptable bleeding in the longer cycle regimens. With regards to bleeding patterns, bleeding days were noted to be decreased in the longer regimens, whereas the number of spotting days increased with longer cycle regimens. Unscheduled bleeding was noted by 16% of women in the 28 day cycle up to 92% of women in the 364 day cycle. Unscheduled bleeding was noted to be a problem by 6% of women in the 28 day cycle, 17% in the 49 day cycle, 39% in the 91 day cycle, and 39% in the 364 day cycle. Adverse events, blood pressure, hemoglobin levels, were comparable in the four treatment regimens.43
An additional prospective study compared bleeding patterns and adverse effects in two groups of women over a seven month period. Women enrolled in the study were of reproductive age and who had been using a 21/7 day combination hormonal contraceptive method for at least 2 months prior to enrollment. Both groups completed a baseline month of CVR use with 21 days with ring and 7 days of a hormone free period and were randomized to one of two groups for six months of continuous CVR use. One group of women replaced the transvaginal contraceptive ring on the same calendar day each month with no ring-free period. The second group of women were instructed to replace the ring monthly. However, the second group was instructed to remove the ring for 4 days if they encountered breakthrough bleeding or spotting for 5 or more days, and then to reinsert the ring for the remainder of the month. Women who used the hormone free interval had a significantly greater percentage of days without breakthrough bleeding/spotting days (95%) compared to the interval-free group (80%).44 This method of extended use, therefore, seems to offer a better solution to an extended ring use with regards bleeding patterns.
Hormonal Side Effects
Daily CVR steroid release rates are usually lower than the daily dose of steroids in OCs. For example, the CVR that releases 120 mcg/day of etonogestrel and 15 mcg/day of ethinyl estradiol is comparable in ovulation suppression and contraceptive activity to the OC containing 150 μg of desogestrel and 30 μg of ethinyl estradiol. Lower doses than OC formulations can be used in CVRs because there is no first-pass effect through the liver with the CVR and because of the constant absorption the bioavailability of the steroid hormones released from the CVR are much greater than oral ingestion of the steroid hormones, despite the lower peak levels of hormones with the CVR. For these reasons, despite the lower steroid doses, the hormonal effects have been similar with CVRs and OCs, both contraceptive and unintended side effects, in all trials. The majority of these are related to irregular bleeding patterns as noted, but other hormonally related symptoms such as nausea, vomiting, headache, and breast tenderness have also been reported with varying frequencies, similar to that found with OCs.
One study attempted to address hormonal side effects by studying insertion instructions. The purpose of this trial was to determine whether the insertion regimen had any effect on hormonal symptoms noted around the time of insertion. Similar to OCs, most hormonal side effects and symptoms related to CVR use occur more frequently at initiation and decrease in frequency or severity with time. This was a cross-over study of a CVR releasing norethindrone and ethinyl estradiol. Three different insertion regimens were studied in 159 women: (1) insertion between 5:00 and 7:00 PM, removed at bedtime and re-inserted in the morning; (2) insertion between 5:00 and 7:00 PM without removal; and (3) insertion between 10:00 PM and 12:00 AM.28 After the insertion, the CVR was worn continuously for 3 weeks, removed for 1 week, and then reinserted using the same schedule for another 4-week cycle. The incidence of any one symptom did not vary by regimen schedule; all treatment groups had the same incidence of symptoms. All symptoms were reported by a minority of subjects. The most common symptom, regardless of insertion regimen, was nausea. Approximately one third of subjects experienced nausea transiently after ring insertion but vomiting occurred rarely. Headaches were reported by less than 15% of subjects with each regimen schedule, similar to the incidence of headaches reported in studies of other CVR formulations.
Hormonally related side effects like nausea, headache, and breast tenderness are associated with all estrogen-containing hormonal contraceptives, including CVRs. Differences in the incidence of these side effects are reported in trials of different CVR formulations. For example, the incidence of hormonal side effects in a large trial of the etonogestrel/ethinyl estradiol CVR was much different from those observed with the norethindrone/ethinyl estradiol CVR. Less than 5% of subjects using the etonogestrel/ethinyl estradiol-containing CVR reported nausea.37 In the comparative trial of the etonogestrel/ethinyl estradiol-containing CVR (NuvaRing) and a levonorgestrel/ethinyl estradiol OC, approximately 2% of subjects in both groups reported hormonally related side effects during treatment.38 In this study, rates of each reported symptom (nausea, breast tenderness, etc) were similar between treatment groups (see Table 3).
Expulsions
Expulsions are uncommon and an infrequent reason cited for discontinuation. Up to 25% of users may experience an expulsion at least one time during CVR use, but a single expulsion does not preclude further CVR use. Expulsion is usually associated with the effort of straining for elimination of stool.45 Neither increased abdominal pressure with coughing, sneezing, or laughing nor vaginal intercourse are commonly associated with CVR expulsion. The size of the CVR is associated with expulsion events. In direct comparison of different sized CVRs, those larger in diameter or thickness are associated with less expulsions.3, 46 Expulsions occurred in fewer than 3% of women using the etonogestrel/ethinyl estradiol CVR in one study.38
Local Effects
Despite their nontoxic, nonirritating, inert material, all CVRs are associated with an increase in vaginal secretions when compared with OCs or to no contraception. This is true for placebo rings as well.3, 46 CVRs and placebo rings cause a weak local inflammatory effect, thereby increasing vaginal secretions. There is no evidence that CVRs increase secretions by altering the vaginal flora or by causing cervical or vaginal infections. After 6 months of use, the number of vaginal leucocytes increased slightly, but the incidence of vaginal infection did not, confirming a local inflammatory effect produced by the CVR.47 Two clinical trials studied the effects of a combination CVR containing levonorgestrel and ethinyl estradiol used for 6 months on the vaginal flora compared with OC users. Neither study found a significant increase in vaginal aerobes, anaerobes, lactobacillus, Candida species, Gardnerella vaginalis, or Neisseria gonorrhoeae colony counts in the CVR users compared with their own baseline evaluation (before treatment) or with oral contraceptive users.47, 48 Thus, use of CVRs does not increase the risk of vaginal infection despite the alteration in vaginal secretions.
Another clinical concern is whether the exposure of the epithelium of the cervix and vagina to the steroid hormones would increase the risk of dysplasia. Fortunately, CVR use is not associated with dysplastic changes in the epithelium of the cervix or vagina.47 In clinical trials, CVR use has not been associated with an increase in the occurrence of cervical lesions. After a preliminary report of the levonorgestrel-containing CVR reported vaginal lesions in approximately 35% of users, further investigation of all CVRs included colposcopic surveillance.12, 49 To prospectively evaluate the colposcopic appearance of the vagina and cervix, colposcopic surveillance was performed at regular intervals in four clinical trials of different CVR formulations with different time intervals: Nestorone alone for 6 months, Nestorone/ethinyl estradiol for 6 months, Norethindrone acetate/ethinyl estradiol for 4 months, or Norethindrone acetate/ethinyl estradiol for 12 months. Colposcopic findings of CVR users were compared both with the subjects' own baseline colposcopic findings (before treatment) and with those of the control group of each trial who underwent the same surveillance. All four trials had the same interval for surveillance and the same reporting criteria; all visible lesions were reported, regardless of any clinical significance. In these trials, there were more vaginal epithelial lesions seen during CVR use than at baseline (17.4% vs. 7.0%). However, CVR users and non-CVR users had the same rate of vaginal epithelial lesions (17.4% vs. 18.0%) during the period of treatment (see Table 4). Baseline comparisons of CVR users and non-CVR users were the same. At the next scheduled evaluation after a vaginal lesion was identified, 75–100% of lesions in all treatment groups had resolved spontaneously. In addition, there was no difference in the incidence of cervical lesions during CVR use compared with baseline evaluation of CVR users or compared with non-CVR users. All follow-up evaluations of ulcerative lesions reported spontaneous resolution. Therefore, CVRs are not associated with any clinically significant cervical or vaginal lesion or dysplasia.
Table 4. Comparison of Incidences of Vaginal Atypical Conditions Among Vaginal Rings Users and Women not Using Ringsa
| Control Groups | ||||
| CVR nonusers | Pretreatment CVR users | Control groups combined | CVR users | |
| Number of women | 107 | 158 | 265 | 169 |
| Number of colposcopies | 317 | 158 | 475 | 507 |
| Colposcopic finding | ||||
| Microulcers | 00.9 (3)0 | 0.0 | 00.6 (3)0 | 1.6 (8) |
| Abrasions | 01.9 (6)0 | 0.0 | 01.3 (6)0 | 1.6 (8) |
| Erythema | 02.5 (8)0 | 0.6 (1) | 01.9 (9)0 | 02.0 (10) |
| Increased vascularity | 00.6 (2)0 | 0.6 (1) | 00.6 (3)0 | 02.6 (13) |
| Ecchymosis | 00.6 (2)0 | 1.3 (2) | 00.8 (4)0 | 0.4 (2) |
| Petechiae | 05.0 (16) | 1.3 (2) | 03.8 (18) | 03.7 (19) |
| Aceto-petechiac | 04.1 (13) | 1.9 (3) | 03.4 (16) | 1.8 (9) |
| White area | 00.3 (1)0 | 0.6 (1) | 00.4 (2)0 | 1.8 (9) |
| Aceto-white areas | 00.3 (1)0 | 0.6 (1) | 00.4 (2)0 | 1.0 (5) |
| Edema | 01.6 (5)0 | 0.0 | 01.1 (5)0 | 1.0 (5) |
| Total atypical conditions | 18.0 (57) | 7.0 (11) | 14.3 (68) | 17.4 (88) |
aPercentage of visits with conditions noted; the number of events is indicated in parentheses.
(Reprinted from reference 44.)
Acceptability
One of the most common concerns about CVRs is whether they will be acceptable to women and their partners. Practitioners and women, after being asked about safety and efficacy of the method, are likely to ask if women will be willing to use a vaginal product for contraception and if it will interfere with vaginal intercourse. This method has been shown to be highly acceptable among different populations.
Investigations of CVRs have been conducted in all types of communities worldwide, in urban and rural areas, and in developed and less developed countries. Continuation rates at 1 year of use have been reported to be as high as 70% but continuation rates are generally less than 50%.37 One year continuation rates for all self-administered hormonal methods of contraception, including OCs, vary among studies but are rarely higher than 70%. In addition, acceptability is not always predicted by continuation rates. The acceptability of CVRs in all studies is high. CVRs are user-controlled and do not require daily or coital attention, contributing to a high acceptability. CVRs are easier to use than other vaginal contraceptive devices. Unlike a diaphragm or cervical cap, CVRs do not need to be fitted for size or specially positioned in the vagina. CVRs are manufactured in one size for each formulation and need only to be in contact with the vaginal epithelium for efficacy. In fact, discontinuation is not usually attributable specifically to the vaginal use of this contraceptive. Inert placebo rings with no hormone were studied for acceptability of the vaginal application of the method apart from the hormonal aspects. Subjects in this study reported that these placebo rings were generally acceptable and easy to insert.3
Both provider and patient experience with the CVR improve the acceptability of the method. A study of CVR use was conducted in both urban and rural areas of two Latin American countries to evaluate acceptability of CVR in a nonrandomized comparison of the CVR with other hormonal methods.50 In this study, women could choose their method of contraception. Women accepted or chose the CVR at much lower rates compared with other hormonal methods of contraception. But this acceptance rate improved over time and was higher at sites where clinic personnel used the ring themselves. In this same study, information was collected about other aspects of acceptability, such as whether women disclosed their method of contraception to others, were comfortable with vaginal placement of the CVR, and whether they removed the CVR for intercourse. This attempted to specifically address the concerns that vaginal administration would be embarrassing for women and that the CVR would interfere with coitus. Vaginal administration did not seem to embarrass or concern the women who chose the CVR. The majority of CVR users, 87.7%, told their relatives or friends that they used a CVR. Although the women were never instructed to check the placement of the CVR, 33–86% of users at different sites reported that they digitally examined themselves to check placement of the CVR.
The CVRs did not appear to interfere with vaginal intercourse. CVRs can be removed for up to 3 hours for intercourse without diminishing their efficacy, but in this study only 23.8% of women removed the CVR for intercourse. Furthermore, only 10–25% of women reported that their partners objected to the CVR during intercourse. A study of the etonogestrel/ethinyl estradiol-containing CVR, which is smaller in diameter (outer diameter: 54 mm; inner diameter: 50 mm) and approximately one-half the thickness of the other CVR, confirmed this finding that the CVR did not interfere with intercourse if left in place. The majority of subjects that used the etonogestrel/ethinyl estradiol CVR, regardless of whether they discontinued use of the CVR early, reported that they and their partners did not feel the CVR during intercourse and did not object to the CVR if it was felt.37 Eighty-seven percent of women who completed the study and 72% of women who discontinued early reported that they did not feel the CVR during intercourse. Seventy-four percent and 58%, respectively, reported that their partners did not feel the CVR during intercourse. The majority of those who completed and discontinued early, 95% and 80%, respectively, did not object to the ring being in place during intercourse. Furthermore, whether women completed the study, they found this method highly acceptable. Ninety-seven percent of women who completed the study and 67% of women who did not reported that they would recommend this method of contraception to a friend.
Potential Future Uses
One study has looked at the feasibility of using a Nestorone-ethinyl estradiol CVR for emergency contraception. This Phase I trial of forty-eight women who were protected from pregnancy using a non-hormonal contraceptive method divided women into three groups based on the size of the dominant follicle at the time of CVR insertion - 12-14 mm, 15-17mm, >/= 18 mm. The ring was kept in place for seven days. Parameters monitored consisted of follicular growth, estradiol, progesterone, LH, FSH levels in the five days after ring insertion. Overall, 87.5% (42/48) women showed anovulation or ovulatory dysfunction during the five day period after CVR insertion vs. 39.6% (19/48) women in the control group of women who took two placebo pills 12 hrs apart. Nausea (12/48), vaginal discharge (10/48), abdominal pain (9/48), and headache (6/48) were the most common adverse effects reported in the CVR group.51 This study introduces the possibility of future use of CVRs as emergency contraception which may be continued for contraceptive coverage through the remainder of a woman's cycle.
CONCLUSION
Family planning and contraception help to reduce maternal morbidity and therefore are considered preventive medicine. In all therapies related to preventive medicine, whether contraception, treatment of hypertension, or prevention of osteoporosis, daily therapeutic regimens are very difficult for patients to maintain. Most current preventive medicine strategies attempt to simplify the requirements of the therapeutic regimen with dosing at less frequent dosing than daily intervals. Contraception viewed from this perspective should be no different. Therefore, new hormonal methods of contraception that are user-controlled, reversible, and do not require daily or coital attention are distinct improvements in contraception for women.
In addition to the advantages mentioned related to use, CVRs have other advantages over OCs. After reaching a steady state, CVRs achieve stable circulating serum levels of steroids during use in contrast to the fluctuating levels that occur with OC use (see Fig. 1). In addition, the absorption of steroids across the epithelium directly into the systemic circulation avoids first pass effect of liver metabolism that occurs after ingestion of steroid hormones. Therefore, even with the lower dose of the contraceptive steroids in the CVR compared with the OCs, the bioavailability (area under the curve) is greater with the CVR. With these pharmacologic advantages, CVRs are able to effectively suppress ovulation (their main mechanism of action), thicken cervical mucous and suppress the endometrial lining with lower steroid doses than OCs. The secondary contraceptive effect on the cervical mucus also contributes to the efficacy of CVRs, particularly CVRs containing a progestin without estrogen. In addition, despite the lower steroid dose, steady level of steroid release with CVRs also provides better bleeding control than low-dose OCs.38
The progesterone-containing CVR, available in some Latin American countries, is appropriate for lactating women, with proven contraceptive efficacy and safety for both the lactating mother and the nursing child. CVRs containing both ethinyl estradiol and a progestin are highly effective contraception, suppressing ovulation in the majority of cycles and achieving a low incidence of breakthrough bleeding (see Figs. 3 and 4). Adverse changes in serum lipid levels have been prevented by using a more potent estrogen, ethinyl estradiol, instead of estradiol with the progestin. This steroid change has not affected contraceptive efficacy or bleeding patterns. In general, CVRs are accepted by users and their partners. Continuation rates of CVRs in clinical trials are similar to other user-controlled contraceptives, like OCs. The schedule for CVR use, whether continuous or cyclic, is more convenient than ingesting a pill every day. Failure to take an oral contraceptive pill each day on schedule results in typical use failure rates of approximately 7% in the first year of oral contraceptive use.52 Now that the combined CVR, Nuvaring, is available in the United States, women have access to an additional effective, long-term, reversible, safe method of contraception that is female-controlled, provider-independent, and coital-independent in use. Future development of combined CVRs will contain other progestins, like Nestorone. Further developments of the CVR should address longer duration of use (up to 1 year) and/or less frequent withdrawal bleeds (every 3 months or complete amenorrhea). A CVR that has dual purposes should also be developed, such as combining postcoital contraception with long-term contraception in a CVR, or combining hormonal contraception with a vaginal microbicide for the prevention of sexually transmitted infections.
REFERENCES
Macht D: The absorption of drugs and poisons through the vagina. J Pharmacol Pathol 10:509, 1918 |
|
Dzuik P, Cook B: Passage of steroids through silicone rubber. Endocrinology 78:208-211, 1966 |
|
Roumen F, Dieben T: Clinical acceptability of an ethylene-vinyl-acetate nonmedicated vaginal ring. Contraception 59:59-62, 1999 |
|
Timmer C, Apter D, Voortman G: Pharmacokinetics of 3-keto-desogestrel and ethinylestradiol released from different types of contraceptive vaginal rings. Contraception 42:629-642, 1990 |
|
Landgren B, Johannisson E, Masironi B et al: Pharmacokinetic and pharmacodynamic Investigations with vaginal devices releasing levonorgestrel at a constant, near zero order rate. Contraception 26:567-585, 1982 |
|
Jackanicz TM: Levonorgestrel and estradiol release from an improved contraceptive vaginal ring. Contraception 24:323-339, 1981 |
|
Mishell DR, Talas M, Parlow A et al: Contraception by means of a silastic vaginal ring impregnated with medroxyprogesterone acetate. Am J Obstet Gynecol 107:100-107, 1970 |
|
Mishell DR, Lumkin M, Jackanicz TM: Initial clinical studies of intravaginal rings containing norethindrone and norgestrel. Contraception 12:253-261, 1975 |
|
WHO: Microdose intravaginal levonorgestrel contraception: A multicentre clinical trial: I. Contraceptive efficacy and side effects Contraception 41:105-124, 1990 |
|
WHO: Microdose intravaginal levonorgestrel contraception: A multicentre clinical trial: IV. Bleeding patterns Contraception 41:151-167, 1990 |
|
Ji G, Hong-zhu S, Gui-ying S et al: Clinical investigation of a low-dose levonorgestrel-releasing vaginal ring. Fertil Steril 46:626-630, 1986 |
|
Bounds W, Szarewski A, Lowe D et al: Preliminary report of unexpected local reactions to a progestogen-releasing contraceptive vaginal ring. Eur J Obstet Gynecol Reprod Biol 1993 |
|
Diaz S, Jackanicz T, Herreros C et al: Fertility regulation in nursing women: VIII. Progesterone plasma levels and contraceptive efficacy of a progesterone-releasing vaginal ring Contraception 32:603-621, 1985 |
|
Chen J, Wu S, Shao W et al: The comparative trial of TCu 380A IUD and progesterone-releasing vaginal ring used by lactating women. Contraception 57:371-379, 1998 |
|
Sivin I, Diaz S, Croxatto HB et al: Contraceptives for lactating women: A comparative trial of a progesterone-releasing vaginal ring and the copper T 380A IUD. Contraception 55:225-232, 1997 |
|
Mishell DR, Moore DE, Roy S et al: Clinical performance and endocrine profiles with contraceptive vaginal rings containing a combination of estradiol and d-norgestrel. Am J Obstet Gynecol 130:55-62, 1978 |
|
Sivin I, Mishell DR, Victor A et al: A multicenter study of levonorgestrel-estradiol contraceptive vaginal rings. I. Use effectiveness Contraception 24:341-358, 1981 |
|
Roy S, Krauss RM, Mishell DR et al: The effect on lipids and lipoproteins of a contraceptive vaginal ring containing levonorgestrel and estradiol. Contraception 24:429-449, 1981 |
|
Robertson D, Alvarez F, Sivin I et al: Lipoprotein patterns in women in Santo Domingo using a levonorgestrel/estradiol contraceptive ring. Contraception 24:469-479, 1981 |
|
Ahren T, Lithell H, Victor A et al: Comparison of the metabolic effects of two hormonal contraceptive methods: An oral formulation and a vaginal ring. II. serum lipoproteins and apolipoproteins Contraception 24:451-467, 1981 |
|
Ahren T, Lithell H, Victor A et al: Comparison of the metabolic effects of two hormonal contraceptive methods: An oral formulation and a vaginal ring. I. carbohydrate metabolism and liver function Contraception 24:415-427, 1981 |
|
Ahren T, Victor A, Lithell H et al: Ovarian function, bleeding control and serum lipoproteins in women using contraceptive vaginal rings releasing five different progestins. Contraception 28:315-327, 1983 |
|
Adams M, Clarkson T, Koritnik D et al: Contraceptive steroids and coronary artery atherosclerosis in cynomolgus macaques. Fertil Steril 47:1010-1018, 1987 |
|
Ballagh SA, Mishell DR, Jackanicz TM et al: Dose-finding study of a contraceptive ring releasing norethindrone acetate/ethinyl estradiol. Contraception 50:535-549, 1994 |
|
Ballagh SA, Mishell DR, Lacarra M et al: . A Contraceptive Vaginal Ring Releasing Norethindrone Acetate and Ethinyl Estradiol. Contraception 50:517-533, 1994 |
|
Weisberg E, Fraser IS, Lacarra M et al: Efficacy, bleeding patterns, and side effects of a 1-year contraceptive vaginal ring. Contraception 59:311-318, 1999 |
|
Weisberg E, Fraser IS, Mishell DR et al: A comparative study of two contraceptive vaginal rings releasing norethindrone acetate and differing doses of ethinyl estradiol. Contraception 59:305-310, 1999 |
|
Weisberg E, Fraser IS, Lacarra M et al: Effect of different insertion regimens on side effects with a combination contraceptive vaginal ring. Contraception 56:233-239, 1997 |
|
Laurikka-Routti M, Haukkamaa M, Heikinheimo O: A contraceptive vaginal ring releasing ethinyl estradiol and the progestin ST-1435: Bleeding control, serum steroid concentrations, serum lipids and serum chemistry. Contraception 42:111-120, 1990 |
|
Alvarez-Sanchez F: Evaluation of four different contraceptive vaginal rings: Steroid serum levels, luteal activity, bleeding control and lipid profiles. Contraception 46:387-398, 1992 |
|
Mascarenhas L, van beek A, Bennink HC et al: Twenty-four month comparison of apolipoproteins A-1, A-II, and B in contraceptive implant users (Norplant(R) and Implanon(R)) in Birmingham, United Kingdom. Contraception 58:215-219, 1998 |
|
Egberg N, van beek A, Gunnervik C et al: Effects on the hemostatic system and liver function in relation to Implanon(R) and Norplant(R): A prospective randomized clinical trial. Contraception 58:93-98, 1998 |
|
Tuppurainen M, Klimscheffskij R, Venhola M et al: The combined contraceptive vaginal ring (NuvaRing) and lipid metabolism: acomparative study. Contraception. 2004 May;69(5):389-94. |
|
Elkind-Hirsch KE, Darensbourg C, Ogden B et al: Contraceptive vaginal ring use for women has less adverse metabolic effects thanan oral contraceptive. Contraception. 2007 Nov;76(5):348-56. Epub 2007 Sep 27. |
|
Timmer CJ, Mulders TM: Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinetics 39:233-242, 2001 |
|
Mulders TM, Dieben T: Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibitionFertil Steril 75:865-870, 2001 |
|
Roumen F, Apter D, Mulders TM et al: Efficacy, tolerability and acceptability of a novel contraceptive vaginal ring releasing etonogestrel and ethinyl estradiol. Hum Reprod 16:469-475, 2001 |
|
Bjarnadottir RI, Tuppurainen M, Killick SR: Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol 186:389-395, 2002 |
|
van den Heuvel MW, van Bragt AJ, Alnabawy AK et al: Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptiveformulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005 Sep;72(3):168-74. |
|
Teal SB, Craven WM: Inadvertent vesicular placement of a vaginal contraceptive ring presenting aspersistent cystitis. Obstet Gynecol. 2006 Feb;107(2 Pt 2):470-2. |
|
Milsom I, Lete I, Bjertnaes A et al: Effects on cycle control and bodyweight of the combined contraceptive ring,NuvaRing, versus an oral contraceptive containing 30 microg ethinyl estradiol and3 mg drospirenone. Hum Reprod. 2006 Sep;21(9):2304-11. Epub 2006 Jun 8. |
|
Fraser IS, Weisberg E, Minehan E et al: A detailed analysis of menstrual blood loss in women using Norplant(R) and Nestorone(R) progestogen-only contraceptive implants or vaginal rings. Contraception 61:241-251, 2000 |
|
Miller L, Verhoeven CH, Hout J: Extended regimens of the contraceptive vaginal ring: a randomized trial. Obstet Gynecol. 2005 Sep;106(3):473-82. |
|
Sulak PJ, Smith V, Coffee A et al: Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring: a randomized controlled trial. Obstet Gynecol. 2008 Sep;112(3):563-71. |
|
WHO: Microdose intravaginal levonorgestrel contraception: a multicentre clinical trial: II. expulsions and removals Contraception 41:125-142, 1990 |
|
Sivin I, Mishell DR, Victor A et al: A multicenter study of levonorgestrel-estradiol contraceptive vaginal rings. II. Subjective and objective measures of effects Contraception 24:359-376, 1981 |
|
Schwan A, Ahren T, Victor A: Effects of contraceptive vaginal ring treatment on vaginal bacteriology and cytology. Contraception 28:341-347, 1983 |
|
Roy S, Wilkins J, Mishell DR: The effect of a contraceptive vaginal ring and oral contraceptives on the vaginal flora. Contraception 24:481-491, 1981 |
|
Fraser IS, Lacarra M, Mishell DR et al: Vaginal epithelial surface appearances in women using vaginal rings for contraception. Contraception 61:131-138, 2000 |
|
Faundes A, Hardy E, Reyes Q et al: Acceptability of the contraceptive vaginal ring by rural and urban population in two Latin American countries. Contraception 24:393-414, 1981 |
|
Croxatto HB, Brache V, Massai R et al: Feasibility study of Nestorone-ethinylestradiol vaginal contraceptive ring for emergency contraception. Contraception. 2006 Jan;73(1):46-52. Epub 2005 Nov 14. |
|
Fu H, Darroch JE, Haas T et al: Contraceptive failure rates: new estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect 31:56-63, 1999 |